| The Generic Infusion Pump (GIP) |
|
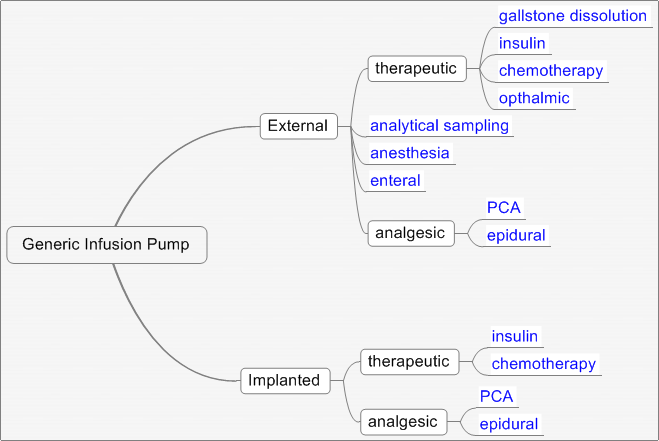
The goal of the Generic Infusion Pump (GIP) project is to develop a set of generic infusion pump (safety) models and reference specifications that can be used as a reference standard to verify safety properties in different classes of infusion pumps. The figure below depicts an infusion pump class diagram with the GIP representing the "base" class.
GIP Class Diagram The purpose of this website is to: a) provide a repository of medical device artifacts for use in projects that advance the science and practice of developing high-confidence medical devices, software, and systems, and b) establish infusion pump safety reference models. As a first step towards accomplishing this purpose, the project provides a preliminary hazard analysis and (safety) reference specifications for a generic PCA (Patient Controlled Analgesic) infusion pump. The archive file contains Simulink® and Stateflow® representations of the PCA pump. This information can be used by researchers and developers interested in the modeling, analysis, implementation, and testing of an infusion pump. We encourage researchers to share corrections, extensions, and research discoveries via this website. Device manufacturers may find this information helpful in terms of verifying aspects of their pump design against the reference model before submitting their product for regulatory review. Manufacturers are particularly encouraged to contribute their knowledge to this effort. We encourage research to extend the GIP into different pump classes as depicted above; for example - volumetric, enteral, and insulin pumps. This information is the result of collaborations between the FDA, UPenn, and Fraunhofer CESE. We encourage others to participate. If interested, please contact us at gipump@seas.upenn.edu. Generic PCAGeneric PCA project consists of three parts: Reference Requirements and Design
GPCA Reference ImplementationAssurance CasesOther infusion pump work (by FDA)PeoplePublicationThe information provided on this web page is for academic research purposes only. We make no claims as to the completeness or correctness of this information. We encourage academics and others to experiment with this work and report their results to this web page so that everyone involved may benefit from the work. Any FDA regulated party using any of this information in their work is responsible for verifying and validating their product as specified in the governing regulations. This project is supported in part by NSF CNS-0610297, "Applying Formal Methods to Improve the Quality of Software in Medical Devices" and NSF CNS-0834524, "Robust Composition and Interoperability of CPS Components". |